Surfactant is typically made up of
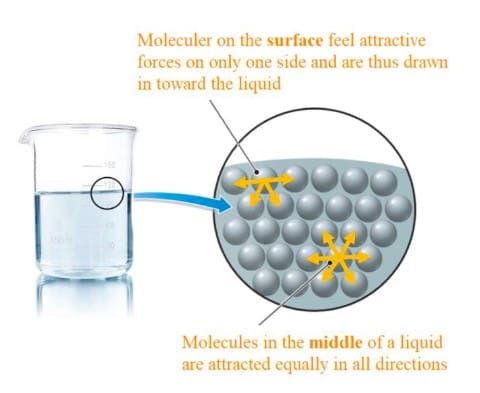
When surfactants, with both polar and nonpolar components, are introduced into a system involving opposing forces (such as air-water or oil-water interfaces), the hydrophilic groups migrate towards the water phase, while the hydrophobic groups move away from water and towards air or oil. This action reduces surface tension because now water can interact with both other water molecules and the hydrophilic (water-attracting) parts of the surfactant.
As a result, water molecules are no longer tightly bound to each other but can spread over a larger area. This effect can be observed when drops of water on a surface lose their dome-like structure upon the addition of a surfactant.

Surfactants are widely utilized, with one of their most common applications being in detergent solutions. Water exhibits relatively high surface tension compared to other liquids. Hence, in cleaning processes, it is crucial to lower surface tension so that water can spread and effectively wet surfaces, allowing it to adhere to and dissolve impurities for removal. Chemicals that achieve this effectively are known as surface active agents or surfactants, which are often referred to as making water “wetter.”
One of the most commonly employed methods for measuring surface tension involves analyzing the shape of an axisymmetric droplet using drop-shape analysis. In this technique, a pendant drop suspended from the tip of a needle exhibits a shape influenced by both gravitational force and surface tension.
These forces act in opposition: gravity attempts to elongate the droplet, while surface tension endeavours to maintain its spherical form. By quantifying the gravitational force and observing the resultant droplet shape, we can calculate the surface tension of the liquid under examination.

Fill the two beakers with 75mL of water. Add 2mL of detergent into one of the beakers using a Syringe. Stir the detergent solution using a glass rod.
Open the Droplet Lab app on the Computer.
Set the density and needle diameter as 0.998g/ml (Water Density) and 1.8mm (Needle used), respectively.
Fill the syringe with water. Lock the syringe in the syringe holder once it is filled. Tip: Make sure the position of the tip of the needle is in the middle of the live window of the screen as shown below.

Slowly generate a drop with the syringe. The ideal situation for drop measurement is to aim for when the drop is just about to detach from the needle tip.
Adjust the image quality (including light condition and focus distance) using the two filters in the right-hand top corner. Once we have a suitable image click a picture by pressing the orange button in the center of the bottom edge.
i. Tip: If you cannot focus on the droplet or its blurry please move the syringe holder further from the phone.

Calibrate the image of the drop.
Go into the measurement interface by clicking on the drop image. Drag the horizontal orange line into the middle of the needle.
Drag the 2 vertical red bars to the needle. And then click on the blue calibrate button besides the calculate button. Once the calibration is done, drag the horizontal line towards the contact point between the needle and the droplet.
Hint: Try watching a video of a water balloon popping in slow motion and observe its behaviour
Hint: Try watching a video of a water balloon popping in slow motion and observe its behaviour
Hint: Try watching a video of a water balloon popping in slow motion and observe its behaviour
Hint: Try watching a video of a water balloon popping in slow motion and observe its behaviour
Hint: Try watching a video of a water balloon popping in slow motion and observe its behaviour
Hint: as an example, what would happen if you used the same syringe multiple times
Droplet Lab was founded in 2016 by Dr. Alidad Amirfazli, faculty member at York University, and two of his researchers, Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Dropletlab © 2024 All Rights Reserved.