This is a complete guide to Surface Energy Measurement in (2025). In this all-new guide you’ll learn all about:

Surface energy is a fundamental property in material science that influences a wide range of phenomena such as wetting, adhesion, and coating. Understanding and measuring surface energy is crucial for optimizing processes in industries ranging from pharmaceuticals to aerospace. This article explores this crucial measurement and its multifaceted applications.
Defining Surface Energy measurement
Surface energy is a critical property that quantifies the excess energy at the surface of a material compared to its bulk. This measurement helps us understand how materials interact with each other and with their environment.
Its importance and applications in the industry
Surface energy plays a pivotal role in various industries
Surface energy is a fundamental property in material science that influences a wide range of phenomena such as wetting, adhesion, and coating. Understanding and measuring surface energy is crucial for optimizing processes in industries ranging from pharmaceuticals to aerospace. This article explores this crucial measurement and its multifaceted applications.
Surface energy is the work required to create a unit area of surface. It results from the imbalance of molecular forces at the surface compared to the bulk of the material
Understanding surface energy is crucial for:
Surface roughness, Material Composition, temperature,Contamination and Surface Treatments can all alter Surface Energy.
Surface roughness, Material Composition, temperature,Contamination and Surface Treatments can all alter Surface Energy.
Surface Roughness
Rougher surfaces have higher surface energy as they increase the actual surface area at a microscopic level.

Material Composition
Different materials have varying intrinsic surface energies due to their molecular structure.

Temperature
Surface energy can change with temperature, generally decreasing as temperature increases due to increased molecular motion

Contamination
Contaminants such as dust, oils, or oxides can significantly alter the surface energy, typically reducing it

Surface Treatments

Contact Angle Method
The contact angle method is a fundamental technique for surface energy measurement. It involves placing a droplet of liquid on a solid surface and measuring the angle formed between the liquid and solid interface. The contact angle provides insights into the wettability of the surface, which is then used to calculate surface energy using models like
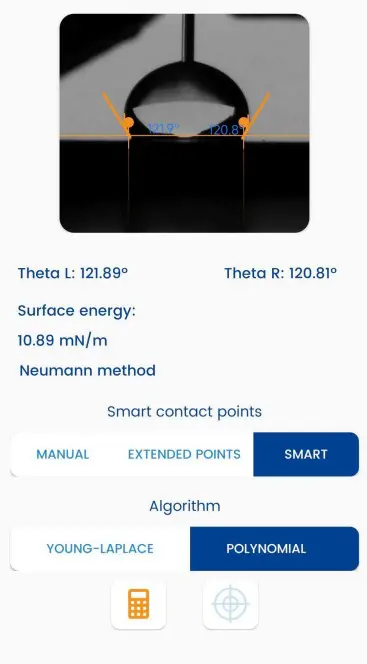
Learn how Surface Energy measurement using Contact Angle method can be done using our Dropometer.
Advantages:
Disadvantages:
Wilhelmy Plate Method
The Wilhelmy Plate method involves dipping a thin, flat plate into a liquid and measuring the force exerted by the liquid on the plate. This force is directly related to the surface energy of the liquid and solid interface.
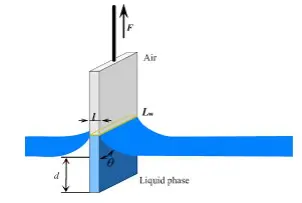
Advantages:
Disadvantages:
Sessile Drop Method
The Sessile Drop method is an automated variation of the contact angle method. It involves placing a droplet on a surface and using automated systems to capture and analyze the droplet shape and contact angle.
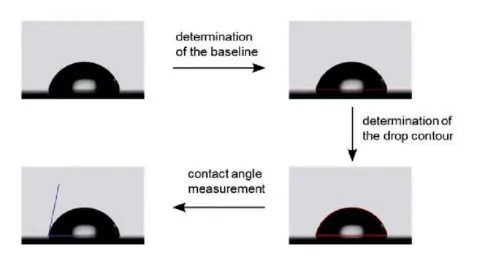
Advantages:
Disadvantages:
Pendant Drop Method
In the Pendant Drop method, a droplet of liquid hangs from a needle, and its shape is analyzed to determine surface and interfacial tension. The technique provides detailed information about the liquid’s properties.
Advantages:
Disadvantages:
Inverse Gas Chromatography (IGC)
Inverse Gas Chromatography measures the interaction between a gas-phase probe molecule and a solid surface. It is particularly useful for analyzing the surface energy of powders and fibers.
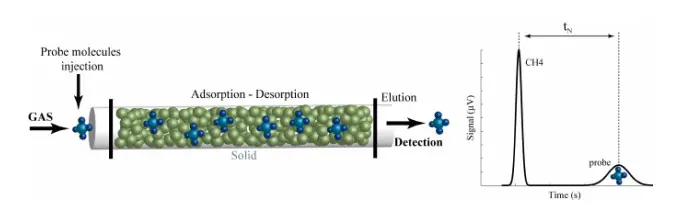
Advantages:
Disadvantages:
Each surface energy measurement method has unique advantages and disadvantages. The Contact Angle and Sessile Drop methods are suitable for smooth surfaces with quick results but may require careful calibration and multiple liquids. The Wilhelmy Plate method is ideal for high-energy surfaces but needs precise alignment. The Pendant Drop method offers accuracy for various liquids, while Inverse Gas Chromatography provides detailed analysis for powders and fibers, albeit with a complex setup. Choose the method that best fits your specific application needs.
Surface Energy Measurements are utilized across various industries. Here are some examples of their applications in each field:


In the dynamic and ever-evolving chemical industry, achieving a uniform dispersion of nanoparticles is a challenging task that often determines the effectiveness of a formulation. Imagine a scenario where nanoparticles, commonly used to enhance the performance or appearance of a product, tend to aggregate, leading to non-uniform distributions within the formulation. This aggregation not only reduces the product’s efficacy but also poses challenges in the manufacturing process.
By precisely manipulating surface properties such as wettability and surface energy, nanoparticles can achieve a homogeneous dispersion throughout the formulation. This uniform dispersion is crucial for ensuring consistent product quality and performance. The benefits of this precise control go beyond achieving uniformity. Improved nanoparticle dispersibility enhances product stability, shelf life, and overall effectiveness, providing a significant competitive advantage in the market.



Imagine you’re a chocolatier, striving to create chocolates that not only taste exquisite but also have a captivating aesthetic. The technique of chocolate tempering is crucial for achieving the desired texture and glossy appearance. Traditionally, tempering requires precise temperature control, but surface science measurements simplify this process significantly.
By accurately measuring surface tension and surface energy, you can attain the optimal temper for chocolates. Manipulating these surface properties ensures your chocolates have a rich, pleasing texture and an appealing, shiny appearance that attracts consumers. Say goodbye to the inconsistencies of traditional tempering methods and embrace a more reliable and efficient approach that elevates the quality of your chocolate creations to new heights.

Challenge: Rare earth element extraction involves complex separation processes dependent on surface interactions.
Solution: The interactions between the mineral surfaces and chemical reagents used in the separation process are influenced by the surface energy. For example, rare earth elements (REE) often exist in complex mineral matrices with other elements. For the selective extraction of REE, chemical reagents are used. Miners can optimize the surface energy values so that these reagents can effectively adhere to the mineral surfaces containing REEs.
Similarly, surface energy optimization can be very useful in the selective extraction of minerals. By modifying surface energy, it’s possible to make the mineral surfaces more or less attractive to specific reagents, thus promoting the selective attachment of reagents to REE-bearing minerals while repelling unwanted minerals.



Challenge: A ship painting company faced uneven surface coatings due to the coating fluid’s viscosity, surface tension, and the substrate’s contact angle.
Solution: The company’s engineering team discovered that using a coating liquid with a contact angle less than 90° caused a pinning effect, reducing surface unevenness. By adjusting the contact angle to create this effect, they mitigated the impact of uneven coatings, leveraging the interplay between fluid viscosity and the substrate’s surface energy.


Even minor contaminants like dust, oils, or residues can significantly alter surface energy readings. These contaminants can create inconsistent contact angles, leading to erroneous calculations.
Solution: Ensure thorough cleaning of surfaces and perform measurements in controlled environments to minimize contamination.
Variations in surface roughness can affect how liquids spread and interact, impacting contact angle measurements. Rough surfaces can lead to inconsistent readings due to uneven wetting.
Solution: Use smooth, uniform surfaces for accurate measurements. If rough surfaces are unavoidable, apply models that account for roughness effects.
Temperature changes can influence molecular interactions at the surface, affecting surface energy. Different temperatures can lead to variations in measurement results.
Solution: Maintain a consistent temperature during experiments and calibrate instruments to account for temperature variations.
Different test liquids interact uniquely with surfaces. The polarity, viscosity, and surface tension of liquids can affect contact angle measurements and, thus, surface energy calculations.
Solution: Select appropriate test liquids that match the material’s properties and ensure they are pure and free from contaminants.
Precise calibration of measurement instruments is crucial.
Misalignment or improper calibration can introduce significant errors
into surface energy readings.
Solution: Regularly calibrate instruments according to manufacturer
guidelines and perform routine checks to ensure accuracy.
Interpreting surface energy data involves complex calculations and models, such as Fowkes, Neumann, and Oss and Good methods. Misapplication of these models can lead to inaccurate results.
Solution: Use software tools for data analysis and ensure a thorough understanding of the theoretical models applied.
Ensuring reproducible results can be challenging due to variations in experimental conditions, measurement techniques, and operator handling.
Solution: Standardize procedures, maintain consistent experimental conditions, and use detailed documentation to improve reproducibility.
By addressing these challenges, researchers can obtain more reliable and meaningful surface energy measurements, enhancing the quality of their scientific and industrial applications.
Ensure Cleanliness:
Control Environmental Conditions:
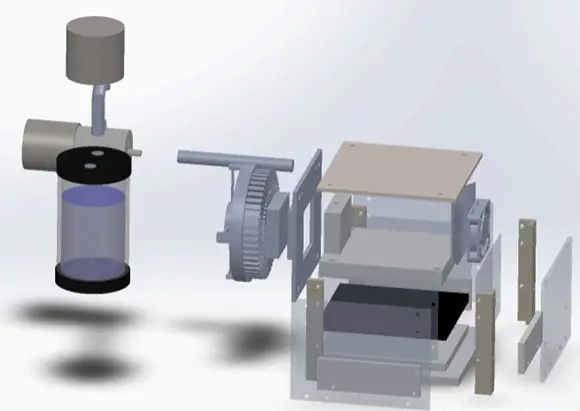
Figure: Design concept of temperature and humidity control chamber by Droplet Lab
Prepare Uniform Surfaces
Droplet Lab’s smartphone-based approach offers comparableSurface Energy measurement accuracy as traditional instruments, along with simplicity, compactness, and portability. This innovative method overcomes the challenges posed by smartphone optical zoom by utilizing an advanced image analysis algorithm.
To measure Surface Energy, The smartphone instrument uses both Young-Laplace and polynomial fitting methods to calculate contact angles. However, it employs Otsu’s algorithm to detect the drop profile from digitally zoomed images, ensuring precise contact point identification. For drops with reflections, the algorithm detects changes in the slope of the drop profile to locate contact points. Without reflections, it identifies the point where the slope becomes zero.
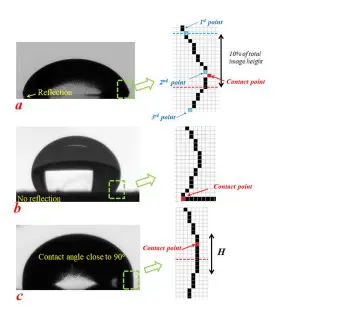
Schematic for principle of contact point detection system: (a) an image of a drop with reflection, (b) an image of a drop without reflection, and (c) a drop with a contact angle close to 90 . The right column shows digitally detected profiles (the dashed box shows the estimated area to guide the eyes).
Handling various drop profiles:
Experimental validation:
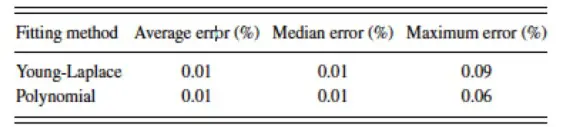
Summary of the error for synthetic contact angle measurements using both the Young-Laplace and Polynomial fitting methods.
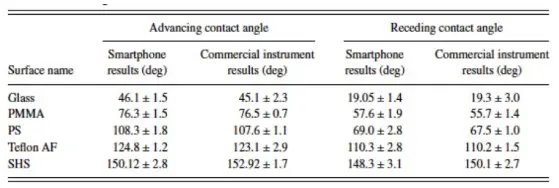
Comparison between measurement results from commercial and smartphone instruments (advancing and receding contact angle measurement). For each of the surfaces, three different drops were used. The reported values are the average value of three measurements.
For more detailed information please refer to the paper published by our founders in AIP Publishing – Review of Scientific Instruments. The user is provided the option of Neumann, Fowkes or Oss and Good method, where the calculated contact angle and other known values are used to calculate the surface energy.
Educational and practical applications:
High-Resolution Method for Nanoscale Surface Energy Measurements
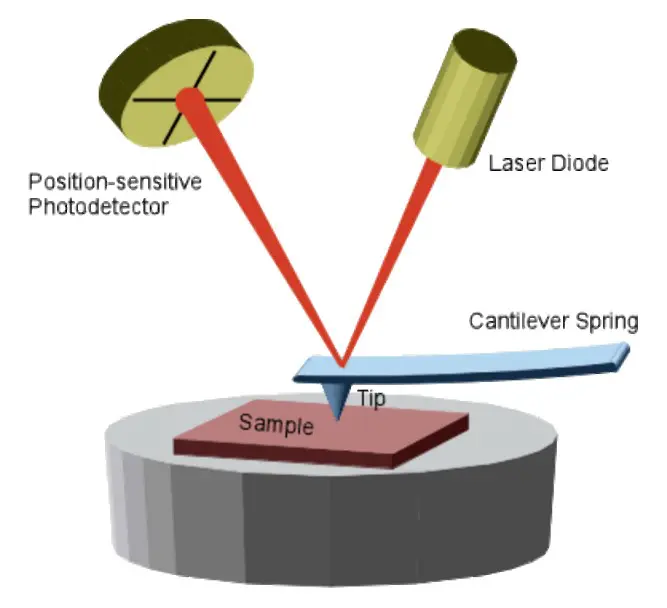
Advantages:
Disadvantages:
Measuring and Analyzing the Energy at Interfaces Between Two Different Phases
Interfacial tension is a critical parameter in systems involving two immiscible phases, such as liquid-liquid or solid-liquid interfaces. The measurement of interfacial tension provides insights into the stability and behavior of emulsions, foams, and coatings. Techniques such as the pendant drop method and spinning drop tensiometry are commonly used to measure interfacial tension. Understanding interfacial energy is crucial for applications in colloidal science, pharmaceuticals, and materials engineering, where control over phase interactions can influence product performance and stability.
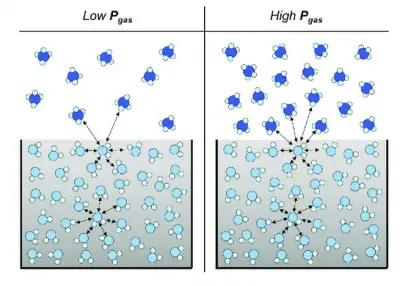
Advantages:
Disadvantages:
Methods to Alter Surface Energy for Specific Applications
Surface modification techniques, such as plasma treatment, chemical etching, and grafting, are employed to alter the surface energy of materials to achieve desired properties. Plasma treatment introduces functional groups to the surface, enhancing hydrophilicity or hydrophobicity. Chemical etching creates micro and nanoscale roughness, modifying wettability. Grafting involves attaching molecules to the surface to tailor its energy. These techniques are essential in industries such as electronics, biomedical devices, and coatings, where specific surface properties are required for optimal performance.
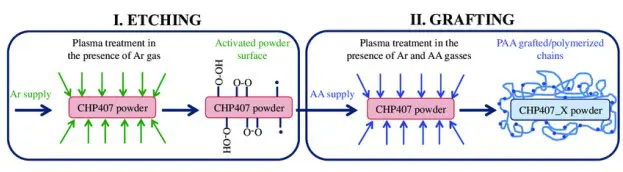
Advantages:
Disadvantages:
Detailed Studies on How Surface Energy Affects Wetting Behavior and Adhesive Properties
Wettability and adhesion are directly influenced by surface energy. High surface energy materials tend to be more wettable and form stronger adhesive bonds. Understanding these properties is crucial for applications such as coatings, adhesives, and biomedical implants. Contact angle measurements are commonly used to assess wettability, while adhesion tests evaluate bond strength. Advanced studies involve examining the interplay between surface roughness, chemical composition, and energy to optimize material performance for specific applications.
Advantages:
Disadvantages:
Exploring Surface Energy in Composites, Biomaterials, and Nanostructured Surfaces
Complex materials, such as composites, biomaterials, and nanostructured surfaces, present unique challenges in surface energy measurement due to their heterogeneous nature. Techniques like AFM, IGC, and contact angle measurements are employed to study these materials. Understanding the surface energy of complex materials is vital for applications in aerospace, biotechnology, and nanotechnology, where surface interactions play a critical role in performance. Advanced studies focus on how the combination of different phases and structures influences overall surface energy and material behavior.
Advantages:
Disadvantages:
By exploring these advanced topics, researchers can gain deeper insights into surface energy phenomena, leading to innovative solutions and improved material performance in a wide range of applications.
Understanding and accurately measuring surface energy is fundamental for advancing material science and various industrial applications. By employing techniques such as the Contact Angle Method, Wilhelmy Plate Method, Sessile Drop Method, Pendant Drop Method, and Inverse Gas Chromatography, researchers can obtain precise and reliable data. Additionally, advanced topics such as Atomic Force Microscopy, interfacial tension analysis, surface modification techniques, wettability and adhesion studies, and the exploration of complex materials offer deeper insights into surface interactions. Through these methods and studies, we can innovate and optimize materials for a wide range of applications, from coatings and adhesives to biomedical devices and nanotechnology
By continuously addressing challenges and employing advanced measurement techniques, we can achieve greater accuracy and reproducibility in surface energy measurements. This comprehensive understanding allows for the development of materials with tailored properties, leading to enhanced performance and new technological breakthroughs.
Droplet Lab was founded in 2016 by Dr. Alidad Amirfazli, faculty member at York University, and two of his researchers, Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Dropletlab © 2024 All Rights Reserved.