Surfactants are an essential class of compounds that play a critical role in reducing surface tension and enabling a wide range of applications in daily life and industrial processes. From cleaning products to personal care items, their versatility stems from their unique chemical properties and behavior at interfaces. This guide dives deep into the science, types, and uses of surfactants, providing a comprehensive understanding of their significance.
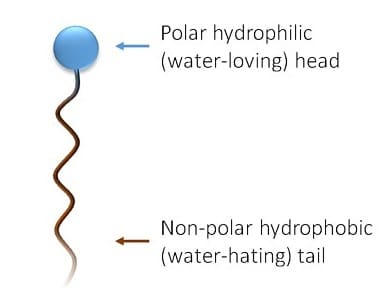

Techniques like the Wilhelmy plate method and pendant drop method are used to quantify how surfactants reduce the surface tension of liquids. These measurements are critical for formulating cleaning products, emulsions, and other solutions requiring specific wetting properties.






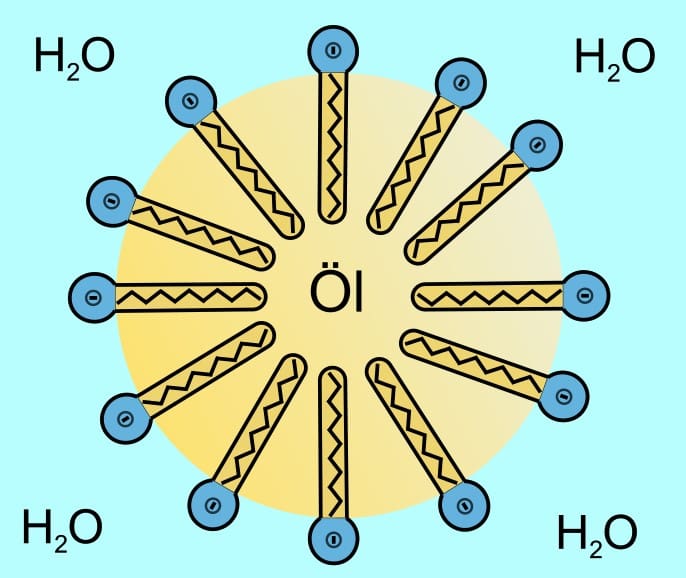
Combining synthetic and natural components, hybrid surfactants aim to balance performance with sustainability. These surfactants are designed to function effectively under challenging conditions, such as extreme temperatures or salinities.

To summarize, surfactants are indispensable in a wide range of applications, from household cleaning to advanced industrial processes. Here are the key points to remember:
Surfactants are much more than just “soap ingredients”—they are the hidden heroes of modern chemistry and daily life. Whether you’re cleaning, conditioning, or innovating, surfactants ensure better outcomes with their versatile, scientifically grounded properties.
Surfactants are remarkable compounds that play a vital role in countless aspects of modern life, from household cleaning to advanced industrial applications. Their ability to reduce surface tension and facilitate interactions between immiscible substances has made them indispensable in industries ranging from personal care to pharmaceuticals. With continued innovation and a focus on sustainability, surfactants are poised to meet the challenges of the future while maintaining their relevance in both established and emerging fields.
Understanding surfactants is key to leveraging their full potential. Whether you’re formulating a new product, studying surface science, or simply curious about the science behind everyday materials, this guide provides a foundation to explore further. As we move toward a greener and more sustainable future, the evolution of eco-friendly surfactants will continue to shape the industries they serve and the world we live in.
Droplet Lab was founded in 2016 by Dr. Alidad Amirfazli, faculty member at York University, and two of his researchers, Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Dropletlab © 2024 All Rights Reserved.