Reviewed: 28
This is a practical guide to Surface Science for researchers working in the Pharmaceutical Industry.
In this all-new guide you’ll learn all about:
Let’s dive right in.

The pharmaceutical industry divides into major segments, including generic drugs, over-the-counter (OTC) medicines, bulk drugs, vaccines, contract research and manufacturing (CRO and CMO), biosimilars, and biologics. Characterizing pharmaceutical powders involves understanding surface properties, which play an important role in processes like liquid penetration into tablets and granules, the spreading of powders in liquids, phase separation, and the formation and stability of emulsions. Additionally, understanding processes such as adsorption, surface tension, and friction at phase interfaces is essential to achieving optimal conditions in pharmaceutics.

We use the important surface properties below to understand the behavior of Pharmaceutical products and improve their quality.
Young – Laplace Method
Polynomial Method
Dynamic Contact Angle
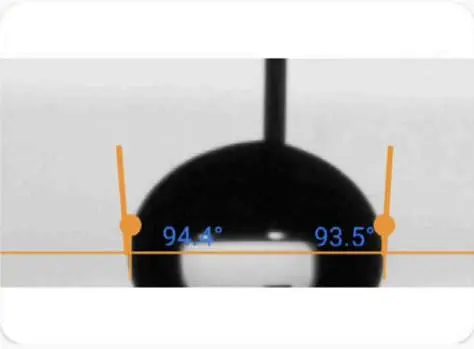
Ideally, when we place a drop on a solid surface, a unique angle exists between the liquid and the solid surface. We can calculate the value of this ideal contact angle (the so-called Young’s contact angle) using Young’s equation. In practice, due to surface geometry, roughness, heterogeneity, contamination, and deformation, the contact angle value on a surface is not necessarily unique but falls within a range. We call this range’s upper and lower limits the advancing contact angle and the receding contact angle, respectively. The values of advancing and receding contact angles for a solid surface are also very sensitive. They can be affected by many parameters, such as temperature, humidity, homogeneity, and minute contamination of the surface and liquid. For example, the advancing and receding contact angles of a surface can differ at different locations.
Practical surfaces and coatings naturally show contact angle hysteresis, indicating a range of equilibrium values. When we measure static contact angles, we get a single value within this range. Solely relying on static measurements poses problems, like poor repeatability and incomplete surface assessment regarding adhesion, cleanliness, roughness, and homogeneity.
In practical applications, we need to understand a surface’s liquid spreading ease (advancing angle) and removal ease (receding angle), such as in painting and cleaning. Measuring advancing and receding angles offers a holistic view of liquid-solid interaction, unlike static measurements, which yield an arbitrary value within the range.
This insight is crucial for real-world surfaces with variations, roughness, and dynamics, aiding industries like cosmetics, materials science, and biotechnology in designing effective surfaces and optimizing processes.
Learn how Contact Angle measurement is done on our Tensiometer
For a more complete understanding of Contact Angle measurement, read our Contact Angle measurement: The Definitive Guide
This property measures the force that acts on the surface of a liquid, aiming to minimize its surface area.
Dynamic Surface Tension
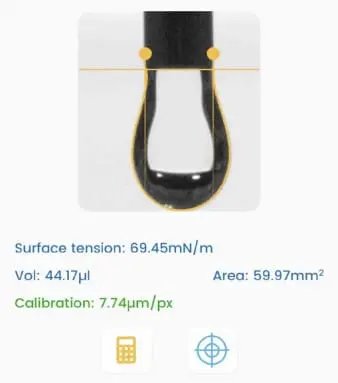
Dynamic surface tension differs from static surface tension, which refers to the surface energy per unit area (or force acting per unit length along the edge of a liquid surface).
Static surface tension characterizes the equilibrium state of the liquid interface, while dynamic surface tension accounts for the kinetics of changes at the interface. These changes could involve the presence of surfactants, additives, or variations in temperature, pressure, and composition at the interface.
Dynamic surface tension is essential for processes that involve rapid changes at the liquid-gas or liquid-liquid interface, such as droplet and bubble formation or coalescence (change of surface area), behavior of foams, and drying of paints (change of composition, e.g., evaporation of solvent). We measure it by analyzing the shape of a hanging droplet over time.
Dynamic surface tension applies to various industries, including cosmetics, coatings, pharmaceuticals, paint, food and beverage, and industrial processes, where understanding and controlling the behavior of liquid interfaces is essential for product quality and process efficiency.
Learn how Surface Tension measurement is done on our Tensiometer
For a more complete understanding of Surface Energy measurement, read our Surface Tension measurement: The Definitive Guide

Learn how Surface Energy measurement is done on our Tensiometer
For a more complete understanding of Surface Energy measurement, read our Surface Energy measurement: The Definitive Guide
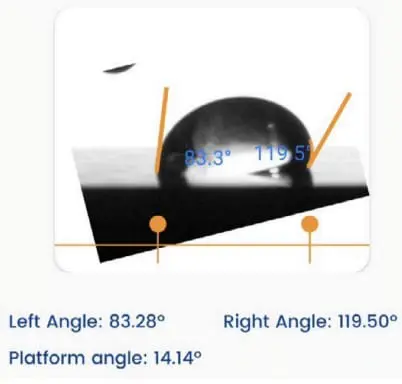
The sliding angle measures the angle at which a liquid film slides over a solid surface. It is commonly employed to assess the slip resistance of a surface.
Learn how Sliding Angle measurement is done on our Tensiometer
For a more complete understanding of Sliding Angle measurement, read our Sliding Angle Measurement: The Definitive Guide
Within the Pharmaceutical industry, several case studies exemplify the advantages of conducting surface property measurements.
Consider a scenario where a pharmaceutical company develops a new oral drug formulation. The drug’s success depends on its ability to dissolve quickly and be absorbed by the body. By measuring the wetting angle of the drug solution on various excipient surfaces, such as the tablet matrix and coating materials, the company can identify which materials promote optimal wetting and dissolution. A lower contact angle indicates better wetting and faster dissolution, leading to improved bioavailability and therapeutic efficacy.


In pharmaceutical manufacturing, ensuring the cleanliness of equipment surfaces is crucial to preventing contamination and maintaining product quality. By measuring the sliding angle of liquids used in manufacturing, the company can identify surfaces that are less likely to allow liquids to adhere. This helps design equipment surfaces that are easy to clean and resistant to liquid adhesion, reducing the risk of cross-contamination and ensuring the production of safe and consistent pharmaceutical products.
Consider a pharmaceutical company developing a transdermal patch for efficient drug delivery. The patch consists of a drug reservoir and an adhesive layer, both essential for optimal drug release and secure skin adhesion. However, the company discovered a discrepancy in the surface energies of these two materials. This insight prompted further investigation into potential causes, such as poor drug adhesion or inconsistent drug delivery. The company meticulously measured the surface energy of both the drug reservoir and the adhesive material, ensuring that these components have matching surface energies for proper bonding and consistent drug release.


Consider a pharmaceutical company developing inhalable medications for respiratory conditions. The effectiveness of these medications relies on producing aerosol droplets of a precise size to effectively reach the lungs. By measuring the surface tension of the liquid formulation used in the aerosol, the company can optimize the spray characteristics to achieve the desired droplet size and uniformity. This process ensures the medication is delivered directly to the target site within the lungs, maximizing its therapeutic effect.
If you are interested in implementing these or any other applications, please contact us.
In an industry where precision reigns supreme, where do Pharmaceutical manufacturers turn to ensure their products can survive scrutiny? The answer lies in standards and guidelines: the compass that guides cosmetics manufacturers through the complex maze of quality and performance.

This standard provides the guidelines for surface treatments which are applicable where the ability of polymer films to retain inks, coatings, adhesives, etc. is being explored. The contact angle of water can be used as a guiding factor to define the effectiveness of surface treatments on polymer films. The water contact angle is measured by capturing an image of a liquid drop placed on a solid and then analysing it. As per the standard, a guiding contact angle range to define the level of surface treatments is given as follows:
|
Marginal or no treatment |
>90° |
|
Low treatment |
85 to 90° |
|
Medium treatment |
78 to 84° |
|
High treatment |
71 to 77° |
|
Very high treatment |
<71° |
This standard provides a test method for measuring the wetting tension of polyethylene and polypropylene films. In this standard surface energy is defined and formulized.

This standard also provides the details for the measurement of the contact angle of water droplets on corona-treated polymer film surfaces and in this way determines the wetting tension of the film. As per this standard, surface energy can be defined as energy that is associated with the intermolecular forces at the interface between two surfaces and it is measured as free energy per unit area. Also static contact angle is defined as an angle between a plane solid surface and the tangent drawn in the vertical plane at the interface between the plane solid surface and the surface of a droplet of liquid resting on the surface.
We hope this guide showed you how to apply surface science in cosmetics industry.
Now we’d like to turn it over to you:
Droplet Lab was founded in 2016 by Dr. Alidad Amirfazli, faculty member at York University, and two of his researchers, Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Dropletlab © 2024 All Rights Reserved.