This is a complete guide to Contact Angle Measurement in (2025). In this all-new guide you’ll learn all about:
So if you’re looking to get an in-depth understanding of Contact Angle measurement, you should get a lot of value from our guide. Let’s dive right in.

Defining contact angle measurement
Importance and applications in the industry

By precisely measuring contact angles, engineers can tailor surface properties to achieve desired functionalities, such as water repellency or adhesion enhancement. In the life sciences, it influences biocompatible surface design for medical devices and implants, ensuring compatibility with biological systems. Moreover, it guides innovations in electronics, energy, and environmental sciences, from self-cleaning surfaces to fuel cell technologies and oil spill remediation strategies.
Wetting describes how a liquid interacts with a solid surface, categorized as complete wetting (θ = 0°), partial wetting (0° < θ < 90°), or non-wetting (θ = 90°).

Wetting phenomena provide insights into material properties and surface behavior. Complete wetting creates uniform coatings, while partial wetting enables controlled-release drug delivery systems. Non-wetting surfaces are fundamental for self-cleaning materials and anti-fog coatings, allowing engineers to tailor materials for specific purposes. Understanding wetting phenomena is a central insight in application and process development.
Surface tension is a fundamental property of a liquid that arises from the cohesive forces between its molecules. It is responsible for the spherical shape of water droplets and the ability of insects to "walk on water." Interfacial tension, on the other hand, is the force of attraction between the molecules at the interface of two immiscible fluids, such as oil and water.
Surface and interfacial tensions are intimately related to contact angle measurement as they influence the wetting behavior of a liquid on a solid surface. High surface tension results in smaller contact angles, as the liquid prefers to spread over the surface—while low surface tension leads to larger contact angles, causing the liquid to bead up. Understanding these tensions helps in predicting and controlling the wetting behavior of liquids on various surfaces.
Contact angles come in various forms, each offering unique insights into surface interactions and essential for optimizing material properties.
Understanding and manipulating these factors holds fundamental importance for processes and applications where surface interactions play a central role.
Needless to say, accurate measurement of contact angles is essential. Various techniques are employed for this purpose, each with its unique advantages and disadvantages. Some of the most common methods include:
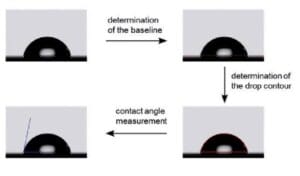
The sessile drop method involves placing a small droplet of a liquid on the surface of interest and measuring the shape of the droplet, particularly its contact angle with the solid, using specialized equipment. We calculate the contact angle by analyzing the drop's size, shape, and equilibrium position. Widely used for its simplicity and accuracy, the sessile drop method is a non-destructive technique suitable for a wide range of materials and applications. However, it relies on nearly perfect spherical droplets, and surface homogeneity is crucial for precise measurements. Despite these limitations, it remains one of the most reliable and efficient methods.
In the sessile drop method, researchers place a small droplet of liquid on a solid surface and determine its angle by analyzing its shape using a contact angle goniometer or other specialized equipment. Maintaining a clean and homogeneous surface and ensuring the droplet remains spherical are essential. This method's simplicity and precision make it a preferred choice.
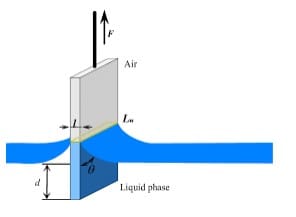
The Wilhelmy plate method involves immersing a thin, flat plate (usually made of glass or metal) into the liquid and measuring the force needed to detach the plate from the liquid, which we then use to calculate the contact angle.
This method is simple and particularly useful for studying contact angles in the context of dynamic wetting. However, it may not offer the same level of precision as the sessile drop method, and ensuring that the plate remains perfectly clean and contamination-free between measurements can be challenging.
The captive bubble method involves immersing a solid substrate into a liquid and introducing a gas bubble into the system. We calculate the contact angle by measuring the pressure difference across the bubble's interface.
This method is suitable for studying contact angles in confined spaces and for specialized applications. However, it is less commonly used due to the complexity of the apparatus and the requirement for precise pressure measurements.

While each method has its pros and cons, the sessile drop method stands out as the most precise and efficient technique for contact angle measurement. Its simplicity and non-destructive nature make it versatile and applicable to various surfaces and liquids, making it highly suitable for both static and dynamic measurements.
Contact Angle Measurements are utilized across various industries. Here are some examples of their applications in each field:

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Automotive industry
Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Aviation & Space industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Biotech industry.
In the dynamic and ever-evolving chemical industry, achieving a uniform dispersion of nanoparticles is a challenging task that often determines the effectiveness of a formulation. Imagine a scenario where nanoparticles, commonly used to enhance the performance or appearance of a product, tend to aggregate, leading to non-uniform distributions within the formulation. This aggregation not only reduces the product’s efficacy but also poses challenges in the manufacturing process.
By precisely manipulating surface properties such as wettability and surface energy, nanoparticles can achieve a homogeneous dispersion throughout the formulation. This uniform dispersion is crucial for ensuring consistent product quality and performance. The benefits of this precise control go beyond achieving uniformity. Improved nanoparticle dispersibility enhances product stability, shelf life, and overall effectiveness, providing a significant competitive advantage in the market.
Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Chemicals industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Consumer Products industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Construction industry

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Cosmetics industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Electrical & Electronics industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Fabric industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Farming & Agriscience industry.
In the beverage industry, maintaining freshness is paramount. For manufacturers of juices, soft drinks, and alcoholic beverages, ensuring product freshness and shelf life is crucial. Conventional packaging techniques often fall short, leading to wasted resources and increased costs.
Accurate contact angle measurement provides a critical evaluation of the wetting characteristics of beverage packaging materials. This knowledge allows you to select materials that effectively prevent moisture infiltration, thereby prolonging the quality and shelf life of your drinks. This practice not only reduces product waste but also lowers packaging costs, ultimately enhancing the financial performance of the organization.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Food & Beverages industry.
Challenge: In the aviation industry, ice formation on aircraft surfaces is a big concern. Ice accumulation on aircraft wings disrupts airflow which leads to reduced lift and control.
Solution: Engineers have worked on an anti-icing systems that depends on contact angles. By carefully controlling the contact angle superhydrophobic surfaces are created. It makes sure that ice cannot easily stick to the aircraft’s wings and surfaces. The new superhydrophobic surface enhanced safety by preventing ice accumulation and reduced the weight and energy consumption associated with traditional de-icing methods.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Mechanical / Industrial sector.
Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Medical Device industry.
Challenge: Achieving efficient flotation in both copper and gold mining is crucial yet complex due to the need for selective attachment of valuable minerals to air bubbles while controlling wetting behavior and surface tension.
Solution: Flotation in mining relies heavily on the interaction between mineral particles and air bubbles. For copper mining, optimizing the contact angle is vital for selectively floating copper minerals and repelling gangue minerals like silica. Achieving an ideal contact angle of 00 ensures hydrophobicity, leading to a high-quality copper concentrate.
In gold mining, controlling surface tension is essential for creating a stable froth. This froth enables gold particles to attach to air bubbles and be separated effectively from gangue materials. Proper surface tension values ensure air bubbles have sufficient buoyancy and stability to carry gold particles to the surface, facilitating efficient recovery.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Mining & Metals industry

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Oil & Gas industry
Challenge: Printing ink in flexible packaging materials can cause contamination in the mechanical recycling process.
Solution: The removal of printing ink residue from the surface of flexible plastic packaging can be achieved through detergency, mechanical, and chemical cleaning processes. In this context, contact angle measurements are invaluable for studying the interaction between the polymer and surfactant. These measurements are highly effective in comparing the wetting behavior of surfactants on various printing ink systems and non-printed film surfaces.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Packaing & Containers industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Paint industry

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Pharmaceutical industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Plastics industry.
Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Semiconductors industry
Challenge: A ship painting company faced uneven surface coatings due to the coating fluid’s viscosity, surface tension, and the substrate’s contact angle.
Solution: The company’s engineering team discovered that using a coating liquid with a contact angle less than 90° caused a pinning effect, reducing surface unevenness. By adjusting the contact angle to create this effect, they mitigated the impact of uneven coatings, leveraging the interplay between fluid viscosity and the substrate’s surface energy.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Shipbuilding industry.
Challenge: Telecom companies face challenges with signal attenuation during heavy rain (rain fade) and disruptions due to ice and snow accumulation on infrastructure like antennas and satellite dishes. These issues can severely impact signal transmission reliability.
Solution: The company aimed to enhance 5G antenna performance under rainy conditions by developing superhydrophobic coatings. Through rigorous experiments with different coatings, they optimized contact angles to design surfaces with high water repellency. This innovation significantly reduced rain attenuation by preventing water droplets from interfering with signal transmission. As a result, the antennas maintained strong signal strengths even during heavy rain.
Moreover, in cold regions prone to ice and snow buildup on satellite dishes, the company conducted tests to identify superhydrophobic materials with large contact angles and low sliding angles. These materials effectively minimized ice adhesion, ensuring uninterrupted signal reception. By reducing the accumulation of ice on the dishes, they enhanced operational reliability and maintained consistent signal transmission in extreme weather conditions.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Telecom industry

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Transportation industry.

Explore other applications of Contact Angle measurement and lots more in our Practical Guide to Surface Science for the Utilities industry.
The importance of calibration
Achieving accurate contact angle measurements requires precise calibration. Subtle variations in an instrument’s performance can significantly impact the reported contact angle. Contamination of the needle with residual liquids, improper droplet size selection, and environmental fluctuations can introduce errors. For example, a clogged needle can distort the droplet shape, leading to an inaccurate contact angle reading. If the droplet size is too small, it limits the resolution of the image analysis software, while an overly large droplet might interact with surface imperfections, skewing the results.
Researchers must employ established calibration procedures and maintain a rigorous schedule for instrument checks to overcome these challenges. This includes verifying the needle’s cleanliness and ensuring its optimal size and shape. Additionally, meticulous calibration with standard reference liquids of known contact angles establishes a baseline for accurate measurements. Regular calibration and maintenance ensure that instruments consistently deliver reliable data.
Limitations in measuring extremely high or low angles
Measuring extremely high or low contact angles presents specific challenges. For very high angles, approaching 180°, droplets tend to form near-spherical shapes, making it difficult to determine the tangent at the contact line accurately.
Conversely, for very low angles, the droplet spreads thinly over the surface, complicating the precise identification of the contact point.

These extreme angles often require specialized equipment and techniques, such as high-resolution cameras and advanced image analysis software, to achieve accurate measurements. Extremely low angles can also be affected by surface heterogeneity and contamination, further complicating measurement accuracy.

Researchers often use advanced techniques like goniometers equipped with high-resolution cameras and image-processing software to address these challenges. These tools allow for more precise determination of the contact angle, even at extreme values. Additionally, specialized techniques, such as sessile drop and captive bubble methods, can be employed to handle these challenging measurements.
Variability due to environmental conditions
Environmental conditions, such as temperature, humidity, and air pressure, significantly impact contact angle measurements.

Temperature variations can alter the surface tension of liquids and the properties of the solid surface, leading to changes in the observed contact angle. Humidity affects the adsorption of water vapor onto the surface, modifying the surface energy and, consequently, the wettability. Air pressure variations further influence the droplet shape and its interaction with the surface. Therefore, maintaining consistent environmental conditions during measurements is crucial for obtaining reproducible and accurate contact angle data.
Researchers must control environmental conditions meticulously to ensure consistent results. This often involves conducting measurements in controlled environments, such as climate-controlled laboratories, and using instruments equipped with environmental control features.

By maintaining stable temperature, humidity, and air pressure, researchers can minimize the impact of environmental variability on contact angle measurements.
Droplet Lab’s smartphone-based approach offers comparable contact angle measurement accuracy as traditional instruments, along with simplicity, compactness, and portability. This innovative method overcomes the challenges posed by smartphone optical zoom by utilizing an advanced image analysis algorithm.
The smartphone instrument uses both Young-Laplace and polynomial fitting methods to calculate contact angles. However, it employs Otsu’s algorithm to detect the drop profile from digitally zoomed images, ensuring precise contact point identification. For drops with reflections, the algorithm detects changes in the slope of the drop profile to locate contact points. Without reflections, it identifies the point where the slope becomes zero
Schematic for principle of contact point detection system: (a) an image of a drop with reflection, (b) an image of a drop without reflection, and (c) a drop with a contact angle close to 90 . The right column shows digitally detected profiles (the dashed box shows the estimated area to guide the eyes).
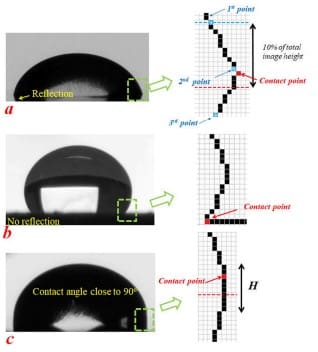


For more detailed information please refer to the paper published by our founders in AIP Publishing – Review of Scientific Instruments.
Superhydrophobic surfaces, characterized by contact angles greater than 150° and low contact angle hysteresis, demonstrate remarkable water-repellent properties.
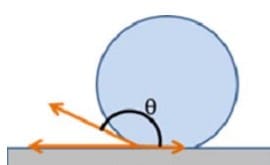
These surfaces mimic the lotus leaf effect, where water droplets roll off, carrying away dirt and contaminants. Conversely, superhydrophilic surfaces have contact angles approaching zero, leading to complete wetting and spreading of water. These surfaces find applications in anti-fogging and self-cleaning technologies. Designing such surfaces often involves creating micro and nanoscale roughness to boost their intrinsic wettability properties
The Wenzel and Cassie-Baxter models describe how roughness and heterogeneity at the nanoscale alter apparent contact angles. The Wenzel state assumes complete wetting of the surface roughness, leading to enhanced hydrophilicity or hydrophobicity depending on the surface chemistry. In contrast, the Cassie-Baxter state involves air pockets trapped beneath the liquid, promoting superhydrophobicity. These nanoscale modifications are vital for developing advanced materials with tailored wettability, such as anti-icing surfaces and biomimetic coatings.
Contact angle measurements provide valuable insights into the wettability, adhesion, and surface energy of materials, impacting fields such as materials science, chemistry, and manufacturing. By utilizing advanced technologies like high-resolution imaging and precise software analysis, these measurements can be conducted with greater accuracy and efficiency.
Looking ahead, advancements in technology will lead to more precise measurements, aided by artificial intelligence. The future holds promise for eco-friendly materials and processes, driven by sustainable practices. From pharmaceuticals to renewable energy, controlling surface interactions will deliver innovative solutions for future challenges.
Droplet Lab was founded in 2016 by Dr. Alidad Amirfazli, faculty member at York University, and two of his researchers, Dr. Huanchen Chen and Dr. Jesus L. Muros-Cobos.
Dropletlab © 2024 All Rights Reserved.